Abstract
Introduction
The phase 3, randomized, open-label TOWER study compared blinatumomab immunotherapy with standard of care (SOC) chemotherapy in adults with Philadelphia chromosome-negative (Ph−) relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (ALL). Blinatumomab significantly improved overall survival as compared with SOC chemotherapy while delaying the time to deterioration (TTD) in health-related quality of life (HRQL), as assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) (Topp et al, Blood 2016;128:222). As reported here, the TOWER study also assessed the presence of ALL symptoms using the newly developed Acute Lymphoblastic Leukemia Symptom Scale (ALLSS).
Methods
HRQL was assessed using the EORTC QLQ-C30 and ALLSS on day 1 (baseline), day 8, day 15, and day 29 in each cycle of investigational therapy, and safety follow-up visit. The HRQL analyses included patients for whom both baseline and ≥1 post-baseline data in any ALLSS or EORTC QLQ-C30 measure have been recorded. ALLSS is a 12-item questionnaire designed to assess ALL-specific symptoms as commonly reported in the literature by adults with R/R ALL. The symptoms assessed were fatigue (2 items), bleeding, bruising, joint/bone pain, fever, frequent infections, lack of appetite, night sweats, shortness of breath, lymphadenopathy, and pruritus (all one item each). In the TOWER study, patients scored the severity or frequency of these symptoms using one of two 5-point response scales (not at all to extremely; never to always), with higher score indicating a worse HRQL. The overall ALLSS score was the sum of the 12 individual item scores (range, 0 to 48).
The mean changes from baseline in overall ALLSS scores and individual item scores were summarized by visit and by treatment arm. A clinically significant TTD in HRQL, or death, was defined for the overall ALLSS score as the time to an increase from baseline of at least half of a standard deviation in the ALLSS score, or death, whichever occurred first. The TTD in HRQL, without the event of death, was assessed in the same manner. For ALLSS individual item scores, the deterioration time to at least one category decrease from baseline, or death, whichever occurred first, was used.
Results
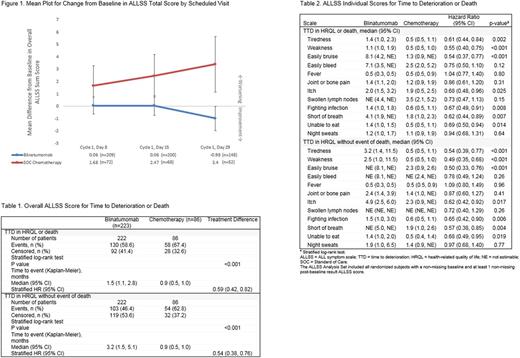
Baseline and ≥1 post-baseline ALLSS scores were recorded for 309 of the 376 patients who received at least 1 dose of study drug (blinatumomab, n=223/267; SOC chemotherapy, n=86/109), and mean baseline scores were comparable between the groups. Patients receiving blinatumomab reported minimal or no change in mean ALLSS total scores during cycle 1 compared with a worsening in mean overall ALLSS scores in the SOC chemotherapy group (Figure 1). This finding is consistent with the previously reported, better posttreatment HRQL observed with blinatumomab across all QLQ-C30 subscales.
As assessed by ALLSS, patients in the blinatumomab arm experienced a clinically significant delay in TTD in HRQL, with or without the event of death, compared with those who received SOC chemotherapy (HR [95% CI] 0.59 [0.42, 0.82] favoring the blinatumomab group [p≤0.001] for TTD in HRQL or death; and 0.54 [0.38, 0.76] favoring the blinatumomab group [p≤0.001] for TTD in HRQL, without the event of death) (Table 1). The TTD in HRQL, with or without death, was longer in the blinatumomab group for all ALLSS items, with the exception of easy bleeding, fever, joint or bone pain, swollen lymph nodes, and night sweats, which were similar in both groups (Table 2).
Conclusions
Consistent with the previously reported HRQL benefit measured using the EORTC QLQ-C30 instrument, the ALLSS 12-item questionnaire found that the use of blinatumomab in patients with R/R Ph− B-cell precursor ALL stabilized HRQL whereas HRQL declined with SOC chemotherapy. When changes in ALL-specific symptoms were observed over time, the TTD in HRQL was delayed in patients treated with blinatumomab relative to patients receiving SOC chemotherapy.
Research supported by Amgen Inc.
Topp: Regeneron: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Other: Travel, Research Funding; Roche: Consultancy, Research Funding; Celgene: Other: Travel; Macrogenics: Consultancy, Research Funding. Zimmerman: Amgen Inc.: Employment, Equity Ownership. Dombret: Chugai/Roche: Consultancy; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; JazzPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding, Speakers Bureau; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sunesis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Stein: Amgen: Consultancy, Speakers Bureau; Stemline: Consultancy. Franklin: Amgen: Employment, Equity Ownership. Nie: Amgen: Employment, Equity Ownership. Cong: Amgen Inc.: Employment, Equity Ownership. Schuh: Amgen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal